添加微信mede1917
或扫描二维码

添加微信mede1917
或扫描二维码

欢迎来到 Medebound 国际医疗平台
平安/泰康签约的优选服务机构
欢迎来到 Medebound 国际医疗平台
平安/泰康签约的优选服务机构


 美联医邦
美联医邦
文章类型: 美国新药和前沿

导读:结肠癌是我国十大常见恶性肿瘤之一,出国看病服务机构了解到发病率呈逐年上升的趋势,但是结肠癌的死亡率相对较低,因此它结肠癌是“幸运的癌”。那去海外看病,结肠癌肝转移如何治疗呢?美联医邦作为拥有9年海外就医服经验的美国本土出国看病服务机构,本篇带大家了解首个印度本土上市的CAR-T疗法NexCAR19。同时分享我们协助国内患者参与美国视频会诊/赴美治疗的案例
海外看病服务机构了解到,根据相关文献报道,有关增强CT或MRI发现的异时性转移病灶,若转移病灶外科手术治疗可行,为了搞清楚病灶侵袭范围,则对选择的病人应进行PET-CT检查。PET / CT用于标示病灶侵袭范围并确定可能的肝外转移灶以排除手术治疗。特别要指出的是,乔伊斯等人报道了术前PET检查改变或排除了25%的准备实时治疗性肝切除手术的患者。最近一项随机临床试验评估了对可切除的异时性转移患者进行PET / CT检查的作用,尽管PET/CT对生存率没有影响,但在行PET / CT后有8%的患者的手术治疗方案发生了改变。
与被诊断的IV期疾病的其他患者一样,应针对肿瘤(转移灶或原发灶)进行KRAS/NRAS基因检测,以确定抗EGFR药物是否可以成为潜在的选择。对野生型KRAS/NRAS基因患者,虽然BRAF基因分型可以考虑,但此项检测目前是可选的,但不是决定是否使用抗EGFR药物的必须检查项目。
推荐多学科联合会诊,出国看病服务机构了解到包括术前请有肝胆和肺转移灶手术经验的外科医生评估。通过对患者化疗史的评估和是否行结肠切除术,异时性转移灶的治疗方案和同时性转移灶是不同的。
根据之前是否接受过化疗,可将可切除病灶的患者进行分类。对于可切除的转移性病灶患者,采用手术联合6个月的围手术期化疗(术前或术后或二者联合),根据前期治疗选择化疗方案。对于无化疗史的患者,首选FOLFOX或CapeOx方案,FLOX、卡培他滨和5-FU/LV作为2线方案选择。在某些异时性病灶患者中,不推荐围手术期化疗。特别指出,既往接受过化疗和手术切除的患者可以进行观察随访,或者疾病进展时给予患者有效的治疗方案,这一原则对于术前肿瘤长大的患者的治疗方案也同样如此(在这些患者中推荐使用生物制剂,2B类证据)。对于之前采用了基于奥沙利铂的治疗方案的患者,首选观察随访。此外,对于接受新辅助治疗的患者,观察随访也是合适的选择。
海外看病专家表示,通过影像学检查确定患有不可切除的病灶的患者(包括那些被认为有潜在可逆性病灶的患者),应根据先前的化疗史,接受积极的全身治疗方案。对于仅有肝转移的患者,可选择HAI治疗(肝动脉灌注化疗,译者注)联合或不联合全身5-FU/LV方案(2B类),该治疗需要在有外科和肿瘤治疗经验的中心进行。接受姑息性化疗的患者应大约每2-3个月进行CT或MRI扫描检查。
1.一线推荐药物:
FOLFIRI或伊立替康;
FOLFIRI +(贝伐单抗[首选]或ZIV-阿普西柏或雷莫芦单抗)
伊立替康+(贝伐单抗[首选]或ZIV-阿普西柏或雷莫芦单抗)
FOLFIRI +(西妥昔单抗或帕尼单抗)*(仅KRAS / NRAS WT)
伊立替康+(西妥昔单抗或帕尼单抗)*(仅KRAS / NRAS WT)
伊立替康 +(西妥昔单抗或帕尼单抗)+威罗菲尼(BRAF V600E正突变)
(纳武单抗或派姆单抗)(仅限dMMR / MSI-H)
2. 二线推荐药物:
伊立替康+(西妥昔单抗或帕尼单抗) *(仅限KRAS / NRAS WT)
瑞戈非尼
三氟尿苷+地匹福林、(纳武单抗或派姆单抗)*(仅限dMMR / MSI-H)
3. 三线推荐药物:
瑞戈非尼
三氟尿苷+地匹福林
以上美国结肠癌肝转移治疗方法,可以帮助海外看病的患者更好地做出明智的医疗决策,美联医邦服务了上千海外国际二诊,美国视频会诊和赴美看病的患者,如有需要请联系我们(400热线:4006162591,电话进来后请告知文章码1219获取专属折扣,客服老师微信:mede1219)
美联医邦海外医疗可协助您参与到美国视频会诊/赴美治疗,详情请阅读下方链接:
美国看病常见问题FAQ
美联医邦提供【病历翻译,医疗签证,医院预约】一条龙服务,为您省时省力,且预约效率更高,可对接到美国医院主任级别专家,费用仅需3万人民币, 请联系我们了解详情。
联系方式:
国内电话热线400-6162591, 电话进来后请告知文章专属码1219获取专属折扣, 或加客服老师微信mede1219立即沟通。
远程会诊|出国成功案例:
「 MD安德森癌症中心口腔癌治疗案例 」 「 MD安德森癌症中心黑色素瘤治疗案例 」 「 MD安德森癌症中心肺癌治疗案例 」 「 MD安德森癌症中心鼻咽治疗案例 」 「 MD安德森癌症中心肠癌肝癌治疗案例 」
免责声明:本文无意影响或改变您的主治医生提供的医疗服务。 请不要在没有先咨询您的主治医疗服务提供者的情况下对您的治疗做出改变。 本文不用于诊断或治疗疾病,也不影响治疗方案。 美联医邦会尽最大努力编辑和更新本页面的信息,但是我们无法保证本页医药信息的精确性和完整性。
美国美联医邦Medebound HEALTH 是一家创始在美国,总部位于纽约,并在大陆和香港设有分部的国际医疗公司。 董事会由资深的美国医院领导组成。 纽约医学院前院长克鲁力博士担任美联医邦董事和秘书长; 纽约五所医院包括纽约最大医院长老会医院董事卡思先生担任美联医邦董事; 哈佛大学医学院教授,飞利浦全球家庭医疗前首席医疗官维特博士 担任美联医邦独立董事。 我们致力于帮助世界有疑难重疾患者对接到美国顶级医疗资源,专注于开展美国肿瘤罕见病名医的第二诊疗意见,视频咨询,出国就医和美国最新药物申请。 至今美联医邦已经签约中国保险集团总部包括中国平安,泰康,太平人寿等,服务覆盖数百万保险人群。

凭借我们30多年的美国医疗网络资源,您可以直接与美国医疗精英对话,在家中咨询美国顶级专家或国际会诊,不出国门获取先进治疗方案。
凭借我们30年的医疗网络资源,您可以直接与美国医疗精英对话。在家中咨询疑难疾病的专家,轻松了解治疗方案。
如需赴美就医或获取海外新药,我们为您一站式安排海外看病等服务。



服务优势
预约知名美国专家譬如安德森癌症医院和梅奥诊所,安排无忧出国看病行程,寻找全球新药新技术医生网络
我们与美国顶级1%的海外医疗医生网络,300+所美国权威医院和药房资源深度合作一站式海外诊疗我们的专属客服医学经理,为您免费咨询,最短时间获取预约优质医疗资源

Jason 王经理
医学客服经理/添加微信:Mede2018

Kiki 圆经理
医学客服经理/添加微信:mede1917
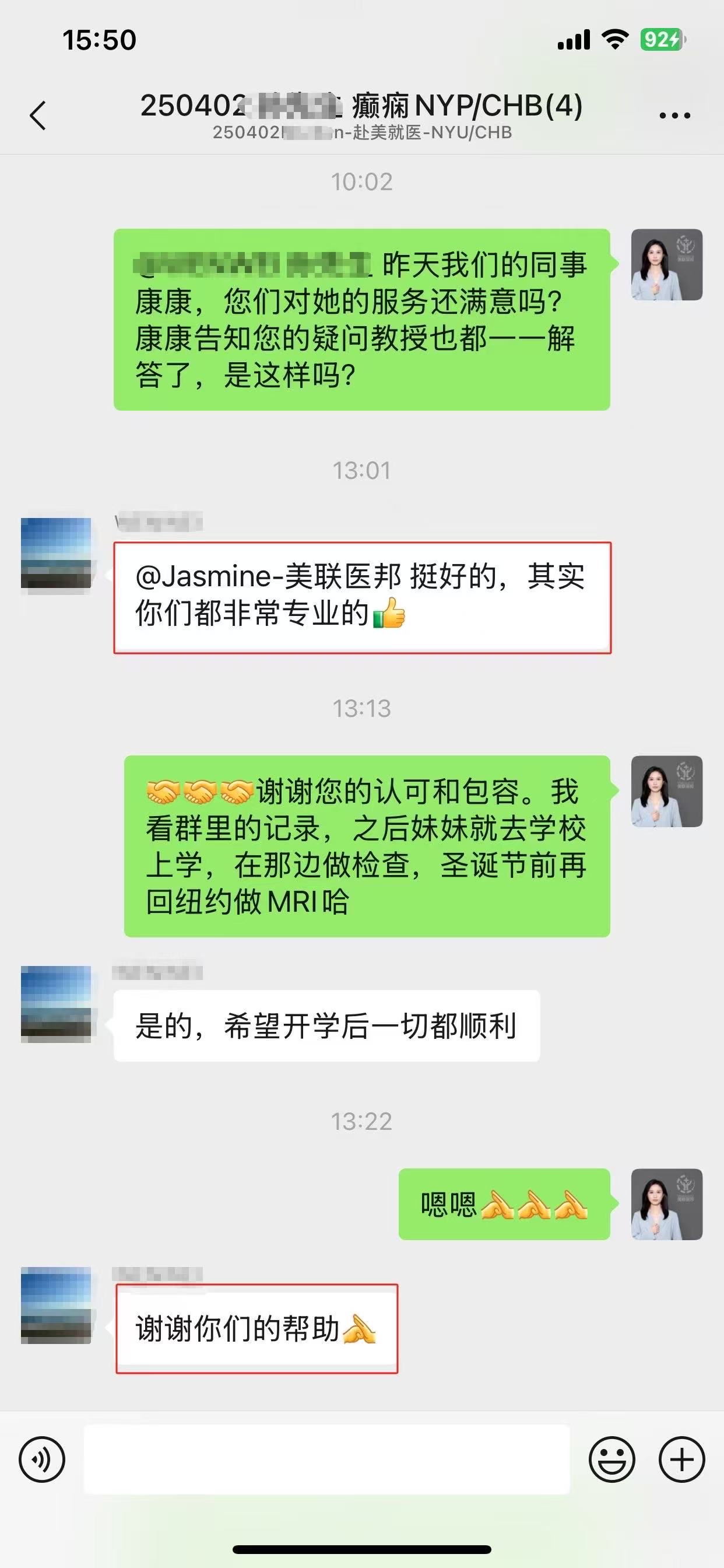
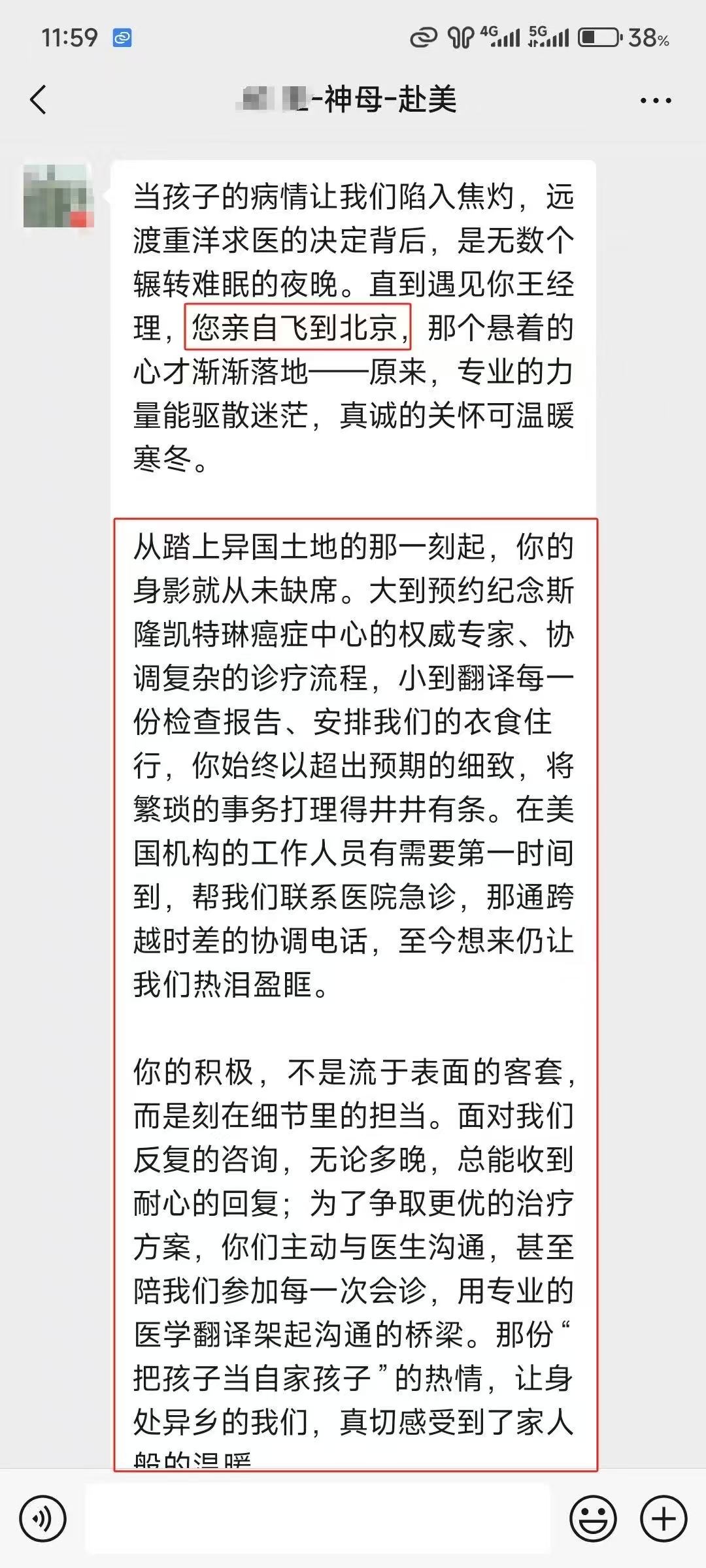

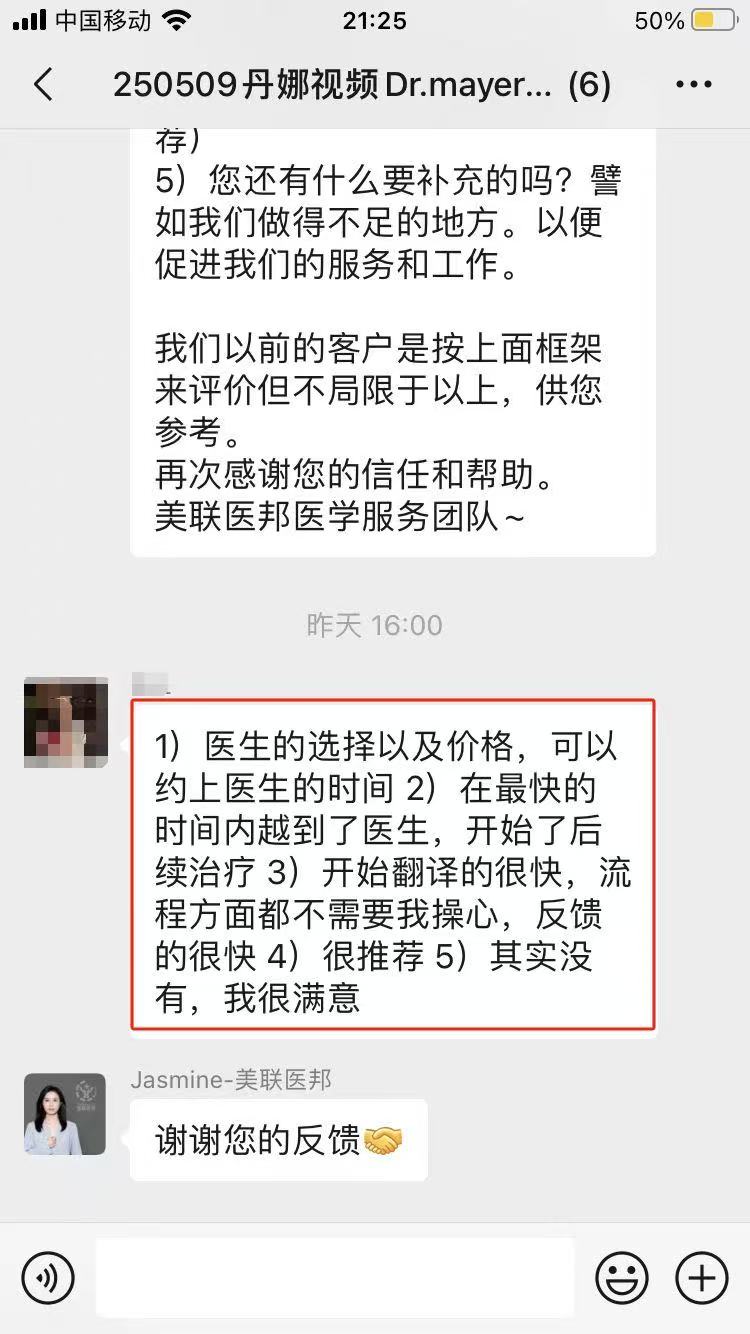
如果您和您的家人有任何医疗和新药需求,请欢迎随时联系我们。我们客服团队工作时间周一到周五早上9点到晚上8点,北京时间。
总部位于纽约,9年专注美国,是赴美就医服务细分领域的头部机构。 始终坚持精英式定制(非保姆式),获30+历史卡思克鲁力医生集团(全美TOP医生联盟)及股东战略产业投资,不涉及风险资本。 作为美国本土医疗资源提供商,美联医邦已与中国平安、泰康人寿、太平人寿等保险集团达成总对总合作。服务覆盖数百万保险客户。美国福布斯榜推荐和英文报道,直通全美前5%顶级专家网络。只精准对接全美72个专科排名TOP3医院,包括梅奥诊所、MD安德森、纪念斯隆-凯特琳癌症中心等百余家美国著名医疗机构。申请美国医院的折扣率10-30%,和美国医院议价能力高。3000+客户的信任选择,一切从用户角度出发,鼓励部分患者远程二诊/问诊拿方案在国内治疗,不过度宣传和劝退不必要的赴美看病。
了解更多美国美联医邦成都运营服务中心
Disclaimer 免责声明:
本文无意影响或改变您的主治医生提供的医疗服务。请不要在没有先咨询您的主治医疗服务提供者的情况下对您的治疗做出改变。本文不用于诊断或治疗疾病,也不影响治疗方案。美联医邦会尽最大努力编辑和更新本页面的信息,但是我们无法保证本页医药信息的精确性和完整性。
纽约总部:
260 Madison Ave 8th Floor #8001,New York, NY 10016
美联医邦Medebound北京国际会诊中心:
北京市东城区天坛南里12号医疗机构
美联医邦Medebound成都运营服务中心:
成都市锦江区红星路一段35号A区1号楼605
(美)+1 917-342-2381
(中) +86 400-616-2591
support@medebound.com